Our science
Two proprietary platforms — V-Body and Chemistry — enabling precise and efficient targeted delivery.
Approach
Unmet medical needs
Despite major advances in the treatment of serious diseases, over 30% of patients worldwide still lack effective therapeutic solutions, leaving millions without adequate care. Current treatments continue to face limitations in terms of efficacy, safety due to limited tissue penetration particularly for complex diseases that are resistant to conventional approaches.
At Valerio Therapeutics, we are developing the next generation of precision-guided therapies to address these challenges in three key areas:
- Immuno-inflammatory and autoimmune diseases, affecting nearly 10% of the global population, or more than 800 million people. Conditions such as rheumatoid arthritis, lupus, and Crohn’s disease remain difficult to treat in a lasting way.
- Rare metabolic diseases, impacting more than 300 million patients worldwide, with over 7,000 identified rare diseases. More than 90% of these conditions have no approved treatment, leaving patients with extremely limited options.
- Solid tumors, which account for 90% of new cancer cases and cause over 10 million deaths per year. Many advanced cancers remain resistant to current therapies, including conventional immunotherapies.
Through cutting-edge technology platforms and a strong commitment to innovation, Valerio is pushing the boundaries of medicine to deliver more effective, precisely targeted, and durable therapeutic solutions.
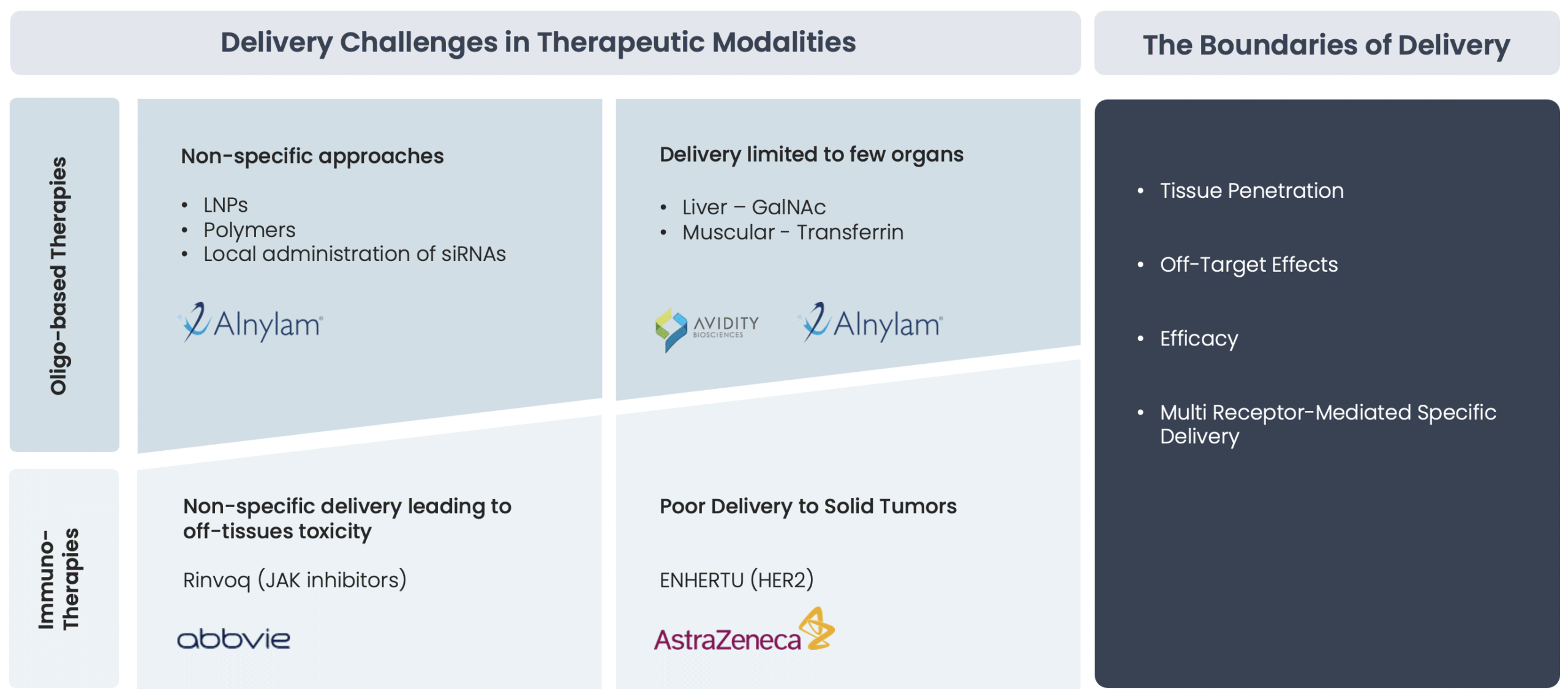
Specific tissue delivery remains a major challenge that impacts both efficacy and safety
One of the most significant challenges in current targeted therapies is the effective and precise delivery of therapeutic molecules to deep-seated tissues. Conventional monoclonal antibodies and other large biologics often struggle with poor penetration, while small-molecule drugs can suffer from limited specificity and off-target toxicity. These limitations lead to:
- Reduced therapeutic efficacy – Incomplete penetration of biologics and suboptimal target engagement of small molecules.
- Increased systemic exposure – Higher doses are often required, leading to potential toxicity and adverse effects.
- Resistance mechanisms – Many advanced cancers and chronic diseases develop resistance due to poor drug distribution and lack of precise delivery.
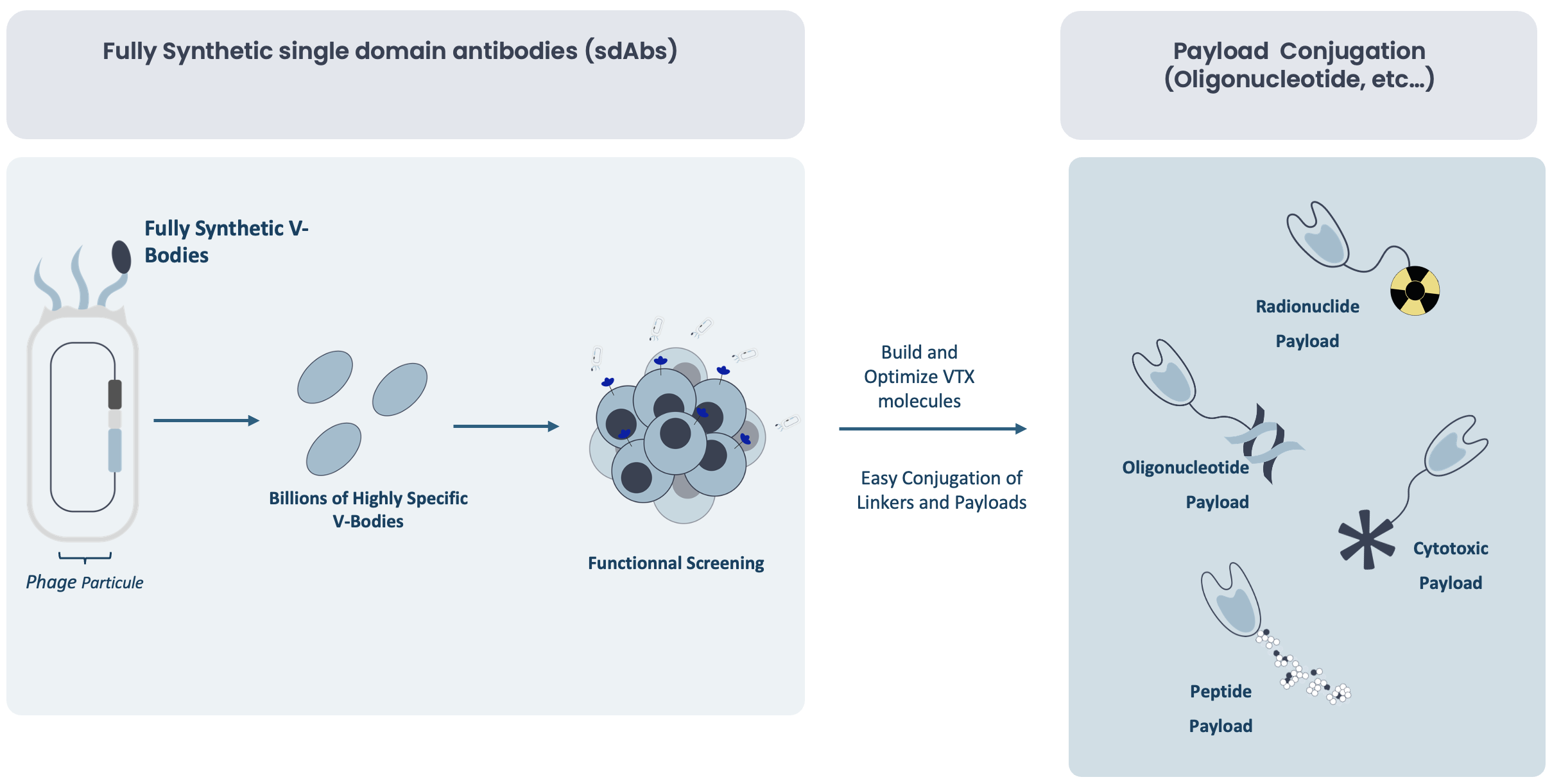
Unique combination of single-domain antibodies with linker/payload chemistry
At Valerio Therapeutics, we have developed a precision-guided approach that integrates advanced biologics (V-Body platform) and innovative chemistry (linker & payload technology) to address these challenges. Our V-Body platform, based on ultra-small single-domain antibodies (sdAbs, ~15 kDa), enables unmatched deep-tissue penetration, while our state-of-the-art linker and payload chemistry allows for customized drug conjugation and optimized release at the target site.